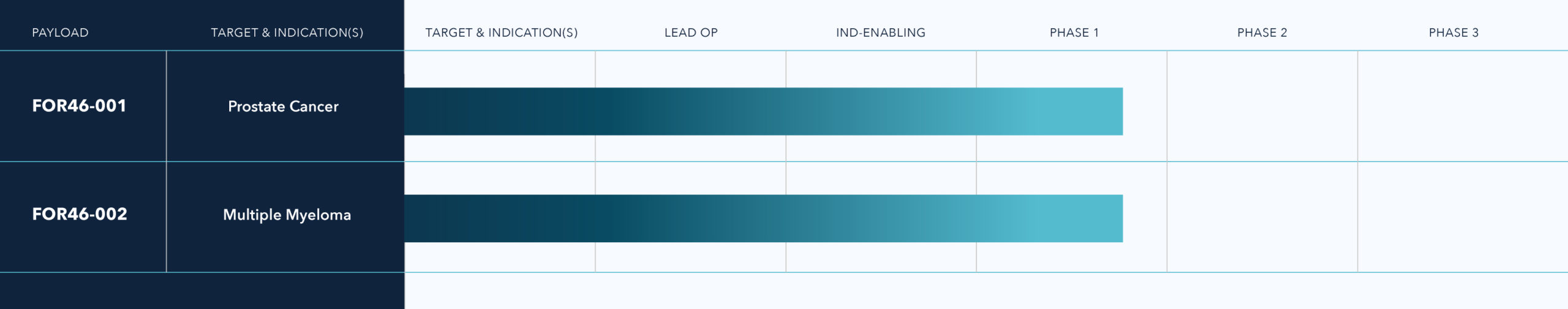
FOR46 is a fully human antibody conjugated to a potent payload, depending on the indication. Fortis Therapeutics is developing FOR46, a novel antibody-drug conjugate (ADC) against CD46, for the treatment of metastatic castration-resistant prostate cancer and late-stage multiple myeloma. The Company is also evaluating additional indications for FOR46.
CD46 is an attractive ADC target for late stage abiraterone or enzalutamide resistant mCRPC:
- CD46 is homogenously expressed in patient samples at very high levels in late-stage PC
- CD46 is further up-regulated following standard of care treatment with abiraterone or enzalutamide
CD46 is an attractive ADC target for MM, especially in patients with a chromosome 1 amplification (1q21) event
- CD46 gene resides on chromosome 1q, which undergoes genomic amplification in ~30% of relapsed MM patients
- The cell surface expression of CD46 is markedly higher on patient derived myeloma cells with 1q amplification
- Pomalidomide increases expression of CD46 and shifts in vitro cytotoxicity by about 1 log
Clinical trials:
For the clinical study in relapsed or refractory multiple myeloma, Fortis has completed single patient cohorts and dose finding and is now enrolling patients in an expansion cohort for treatment with 2.4 mg/kg or FOR46 (ClinicalTrials.gov Identifier: NCT03650491).
For the clinical study in metastatic castration-resistant prostate cancer (mCRPC), Fortis has completed patient enrollment in the dose escalation cohorts (up to 2.4 kg/mg) and is beginning enrollment in dose expansion cohorts in metastatic castration-resistant prostate cancer and additional indications (ClinicalTrials.gov Identifier: NCT03575819).

